The Challenge – industry and academia speak different languages:
- The transfer of academic innovation into drug development requires the translation of “efficacy claims in animals” into “therapeutic efficacy in humans”.
- For most academic projects, a critical step is also to establish a successful collaboration with pharma companies that have the knowledge and resources required to develop novel therapeutics.
- However, pharma and academia often speak different ‘languages’ and even the most promising projects can fail if the alignment on critical next steps and quality measures is ‘lost in translation’.
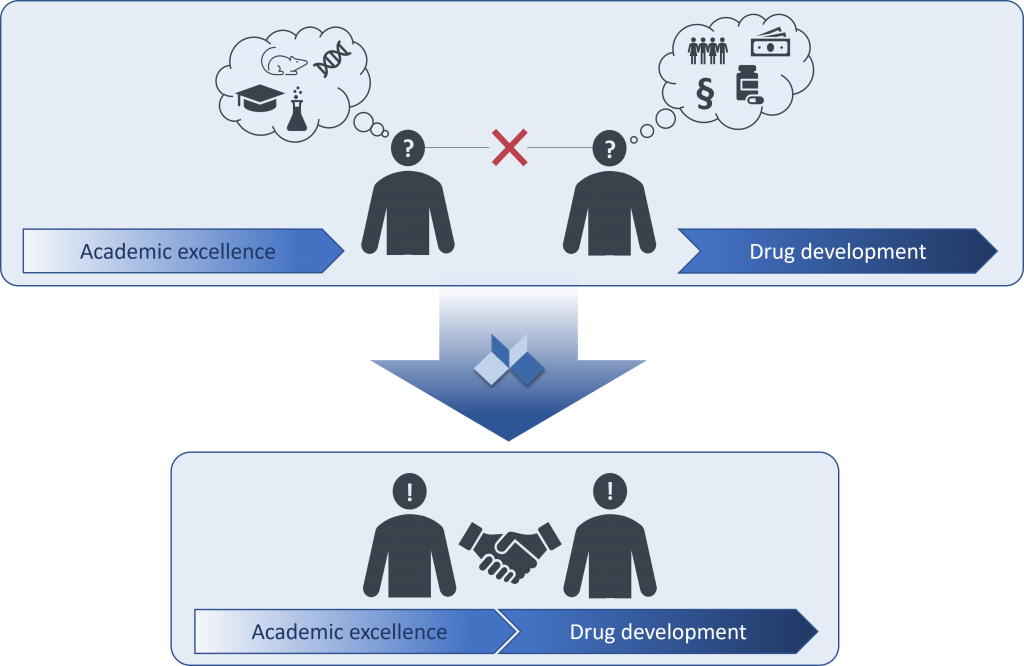
The Solution – engage the PAASP team to facilitate communication and align on expectations:
- Learn about the expectations of potential pharma partners as early as possible (i.e., before designing and conducting key sets of experiments).
- Discuss and prioritize activities that will be essential for decision-making by pharma partners.
- Implement research rigor and data integrity measures to ensure robustness and reliability of experimental findings.
1. At what stage of a project does PAASP get involved?
From target validation to Phase 2-enabling activities.
2. What is the first step to get PAASP engaged?
We welcome an informal way to initiate a discussion by sharing a nonconfidential data package and setting up an introductory tele/videoconference to define project objectives.
3. What types of organizations use project management support by PAASP?
Academic (individual research groups and research centers in general), industry (biotech and established biopharma), and investors.
4. Is PAASP’s support restricted to any specific therapeutic area?
PAASP’s project management focuses on deliverables (rigor in key sets of evidence, data integrity, confidence in decision making) that are shared across all therapeutic areas.
5. What are the costs for getting PAASP’s support?
The costs are discussed individually for each project. PAASP offers a flexible compensation model (ranging from FTE-based contractual agreements to success-based compensation).

Case #1: Academic research center
- Organization: Academic research center
- Objective: Partnership with a pharmaceutical company (co-development, licensing)
- Project stage: Target validation
- PAASP contribution: Work package related to research rigor and data management
- Collaboration model: Joint research grant application (public funder). PAASP acts as a co-applicant / co-investigator (no fees or payments outside the research grant)

Case #2: Academic TTO
- Organization: University technology transfer office (TTO)
- Objective: Spin-off a research project, set up a start-up, attract venture capital funding
- Project stage: GLP tox ready
- PAASP contribution: Gap analysis and advice on data integrity; interim nonclinical development and CMC function
- Collaboration model: Success-based fee (equity)

Case #3: Pharmaceutical company
- Organization: Midsize pharmaceutical company
- Objective: Partnership with another pharmaceutical company (co-development, licensing)
- Project stage: Phase 2-ready
- PAASP contribution: Conduct of mock due diligence
- Collaboration model: Contractual agreement with the service-requesting company

Case #4: Pharmaceutical company
- Organization: Mid-size pharmaceutical company
- Objective: Decision to advance an internal program to Phase 2
- Project stage: Phase 2 ready
- PAASP contribution: Shadow project director
- Collaboration model: FTE-based contractual agreement with the service-requesting company

Case #5: Non-pharmaceutical company
- Organization: Established company in a field unrelated to drug development
- Objective: Out-license a non-core asset
- Project stage: GLP tox ready
- PAASP contribution: External project management, contacts with potential investors, project presentations
- Collaboration model: FTE-based contractual agreement with the service-requesting company

Case #6: Special purpose acquisition company
- Organization: SPAC
- Objective: Acquisition of an asset
- Project stage: Phase 1-ready
- PAASP contribution: Part of a due diligence team, management of additional R&D activities preceding and enabling the investment decision
- Collaboration model: Contractual agreement with the service-requesting company

