For over a decade, there is a trend of less specialized investment firms and public equity investors betting on life sciences companies with high-risk programs without clinical proof-of-concept. This area of investment was previously targeted by investors with knowledge and resources to assess the risks based on adequately planned and performed due diligence. Our analysis of these trends calls for the establishment of a standardized „Home Inspection” procedure that would better inform the early-stage investors about the risks involved, especially related to the robustness and quality of the underlying scientific data sets of newly publicly traded companies in the life science sector.
The constantly changing regulatory environment, ever-growing competition, and risks of technical failures at every stage of development are just some of the threats that make investing in life sciences companies with early-stage assets such a high-risk endeavor.
Yet, the medical need persists and, in many areas, even continues to increase as does the investors’ appetite for innovative projects that may lead to safe, effective and well differentiated products.
The drive for unprecedented mechanisms of action with transformational therapeutic potential adds further risks that are less commonly acknowledged.
In a binary world where we still typically celebrate results whenever a p-value is below the magic level of 0.05, novelty means high risk. Indeed, it is usually not taken into account that the long-shot and good-bet experiments (Nuzzo, 2014) may deliver results with the same p-values despite the likelihood of observing a true effect in the latter being much higher.
If the decision-enabling evidence is not supported by properly designed and adequately powered preclinical studies, then higher technical risk is carried forward into higher cost clinical development programs. Thus, until clinical proof-of-concept (POC)* study results are available, it will not be known whether such risk was justifiable.
To better understand at what stage (i.e. before or after clinical POC) major investment were made, we took the five largest US-based VCs (Fierce Biotech 2013 list) and searched for all exits their portfolio companies made between 2010 and 2015.
We were particularly interested to see how often an exit occurs before clinical POC and whether there is a difference between conventional exits (acquisitions by large biopharma) vs IPOs.
According to our analysis, most exits were before clinical POC and, what was particularly surprising, this was much more likely for companies that went public compared to those that were acquired by large biopharma peers (Figure 1).
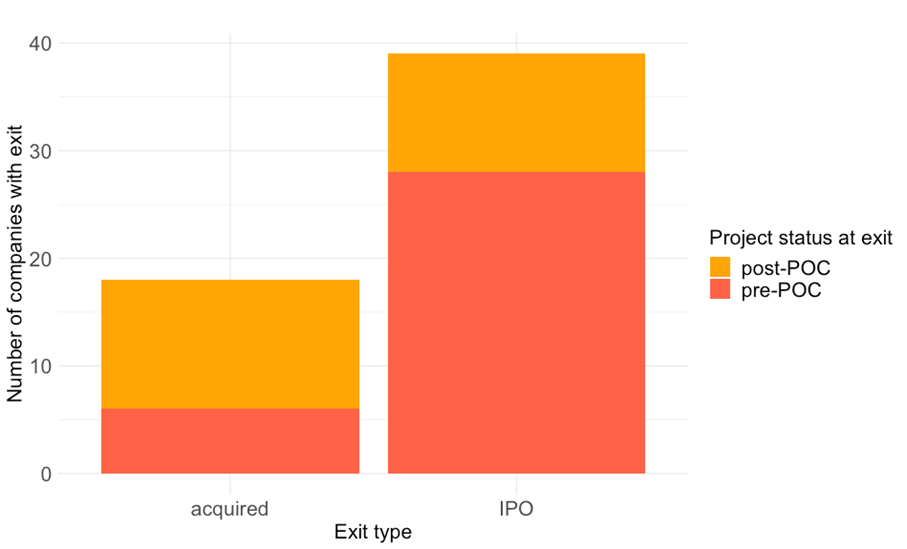
Figure 1: The status of the lead project was explored for 57 life sciences companies that went public or were acquired between 2010 and 2015. Data are shown as the total number of companies per exit type (acquisition vs IPO) and project status at exit (the lead project with or without a clinical proof-of-concept). Please see text for further details.
Importantly, a biopharma company evaluating a pre-POC asset has access to all preclinical data and information generated around the asset and usually engages its R&D and intellectual property legal departments, as well as external experts, to conduct rigorous due diligence assessments. In some cases, biopharma companies would have resources to run experiments to confirm key aspects of the project under evaluation. A well prepared and executed due diligence assessment can certainly mitigate at least some of the risks that are normally associated with early-stage assets.
For public equity investors, however, the situation is very different. They do of course have access to information shared by the biotech team (mostly in the form of corporate presentations or what is disclosed in IPO filing documents), can read analysts’ reports and can engage key opinion leaders to judge on the general scientific validity of the approach taken by a particular biotech. However, not being able to evaluate the actual basis for decisions (original documentation, study protocols, raw data, reports, etc.), public equity investors essentially make blind bets in a sense that the strength of preclinical evidence for advancing the asset into clinical trials remains a black box.
Given that, especially for a publicly traded small biotech companies with a limited number of drug candidates in its pipeline, the price per share directly depends on the fate of its lead compound(s), public equity investors in the life science sector should ideally be able (or empowered) to also evaluate various factors influencing the likelihood of obtaining a positive clinical POC such as: a) internal validity of the decision-enabling preclinical studies, b) the external validity of the decision-enabling preclinical studies and c) the translational validity of the animal models used in these preclinical studies. If one of these factors is weak and insufficient, this will most likely have direct negative consequences for the clinical R&D program (Scannell et al., 2022).
Thus, what can be done to mitigate the risks for public equity investors (including – indirectly – many of us as our pension funds are commonly making such investments)?
First, institutional investors should not rely solely on analysts’ reports and key opinion leaders’ backing of specific projects but rather invest in a data-driven due diligence process focusing on data integrity requirements and the level of internal, external and translational validity of underlying preclinical data sets.
Second, if pre-POC companies aim to attract public money, authorities regulating access to public trade markets (such as the SEC) should request and companies should disclose information about the robustness of critical preclinical evidence regarding their lead assets in their filing documents/company presentations. This can be accomplished via a mechanism similar to what is known as “home inspection” in the real-estate market. In some countries (e.g., in the USA), when a residential house is being purchased, there is usually a legal requirement for a home inspection (due diligence) by an independent home inspector before the prospective buyer actually completes the purchase transaction. Albeit this is a very crude analogy, public equity investment is facing similar risks due to the buyer’s lack of technical insight into the asset quality.
* for the purpose of our analysis, clinical proof-of-concept studies were defined as completed clinical double-blind placebo-controlled (or other comparator, if appropriate) RCTs, designed and executed by or under the supervision of the biotech/biopharma company with the primary endpoint(s) related to therapeutic efficacy (i.e., rather than mechanistic proof of concept)
References:
- Nuzzo, R. Scientific method: Statistical errors. Nature 506, 150–152 (2014).
- Fierce Biotech: The Top 15 Biotech VC firms 2013:https://www.fiercebiotech.com/special-report/updated-top-15-biotech-vc-firms
- Scannell JW, Bosley J, Hickman JA, Dawson GR, Truebel H, Ferreira GS, Richards D, Treherne JM.: Predictive validity in drug discovery: what it is, why it matters and how to improve it. Nat Rev Drug Discov. 21, 915-931 (2022).
0 Comments
Leave A Comment