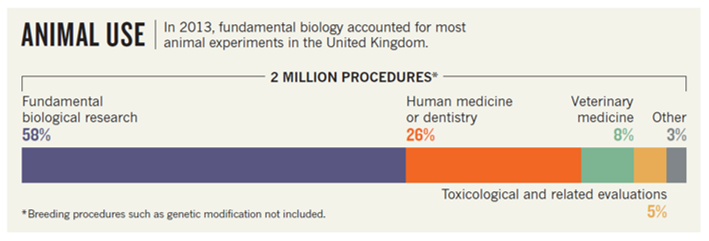
On September 29, 2022, the U.S. Senate passed the FDA Modernization Act 2.0, S.5002, by unanimous consent. While it is unclear whether a companion bill introduced in the U.S. House of Representatives will successfully pass, allowing the legislation to become law, if enacted, this could curb animal testing in the coming years. One of the sponsors of the bill, Senator Dr. Rand Paul, said: “This is the biggest policy development in Congressional history on the fight to replace animal testing with morally and scientifically superior methods. The FDA Modernization Act 2.0 will accelerate innovation and get safer, more effective drugs to market more quickly by cutting red tape that is not supported by current science” (LINK). In the past years, there has indeed been a lot of public discussion about the so called “reproducibility crisis” in science. Among the various consequences of “irreproducibility”, the waste of ethically sensitive resources such as laboratory animals that demands our immediate attention. Does the FDA Modernization Act 2.0 aim to support “morally and scientifically superior methods” that will result in more robust and reliable data across the most affected areas of biomedical research? Likely not. First, the FDA’s rules and regulations are primarily concerned with “regulated” research, leaving decisions about the conduct of most research up to scientists and institutions. Second, as pointed out by Senator Cory Booker, the focus of this Act is preclinical safety assessments: “Thanks to modern scientific innovation, the use of animal toxicity testing for experimental drugs has become increasingly obsolete.” However, as this 2013 UK-based analysis suggests (LINK), this represents a fairly small percentage of the total animals used for research and will likely have a minimal impact on reducing the use of animals across all of research:
In recent years, an increasing number of voices have advocated for expanding the existing 3Rs principles of animal use in research (replacement, reduction and refinement) by introducing Harm-Benefit Analysis (LINK & LINK) or adding three more Rs (robustness, registration and reporting; LINK5). This represents a more logical solution to reducing the use of animals in research given the current state of scientific discovery towards suitable replacement methods. How does regulated toxicology research compare to nonregulated biology and pharmacology research in terms of the Harm-Benefit Analysis or 6Rs assessments? Today, regulated toxicology research delivers overall without any doubt more robust and reliable results than nonregulated pharmacology or biology. Many aspects of Good Research Practice that we wish to see in nonregulated research are a norm in regulated research (completion of detailed study plans prior to study initiation, prespecification of key study characteristics and data analyses, rigorous training of experiments, solid documentation practices, etc.). In the future, by increasing our ability to collect, process and synthesize very large datasets, will be able to accurately model the exposure of every tissue to novel drugs and their metabolites and acquire the knowledge about most or all receptor targets and the mechanisms responsible for safety signals. As a result, there will be a natural transition that allows in vivo methods to be replaced with newer, more dynamic methods such as organs-on-a-chip. But we are not there yet. It is difficult to compare regulated toxicology and nonregulated pharmacology research in terms of the predictive validity of their models. One could perhaps only agree with a statement that false negatives are of greater concern in toxicology studies. Hence, skipping essential parts of a preclinical safety assessment program by replacing animal studies with alternatives of unknown predictive validity exposes human subjects to greater risks than skipping parts of nonregulated pharmacodynamic or biological evidence. Why did we call our commentary “The Steal Syndrome”? Steal syndrome is a condition in which blood is taken from a narrowed artery (e.g., in the heart, due to atherosclerosis) and rerouted to other blood vessels (in other words, “stolen” by other blood vessels). This condition can occur when some medications widen arteries and increase blood flow through healthy “responsive” vessels, thereby worsening the blood supply through less “responsive” vessels. The FDA Modernization Act 2.0 may work like such medications by regulating those parts of the drug development cascade that are “responsive” and thereby worsening the crisis – i.e., human subjects participating in clinical studies will be exposed to more novel drugs with unknown safety risks. The FDA Modernization Act 2.0 is a very important step towards a future free of animal testing, but it should not let us forget that today we are not scientifically positioned to develop novel drugs without animal testing. Thus, more effort is needed to enhance the rigor of currently less “responsive” areas of biomedical research – basic and applied biology and pharmacology – to enable the realization of animal-free testing across all areas of research. By Anton Bespalov (PAASP) and Chantelle Ferland-Beckham (Cohen Veterans Bioscience). The authors thank Dr. Thomas Steckler (Janssen) for a critical review and feedback.
0 Comments
Leave A Comment